Software
Quality Management System
Software
Quality Management System
Streamline quality and exceed customer expectations.
Reduce costs, release products faster and ensure consistent quality
Keep quality, suppliers, and manufacturing under control with one connected platform.
Nulogy Quality Management System (QMS) gives quality managers one workspace to control specs, documents, issues and manage supplier performance. Enforce line checks, capture evidence on any device, and route review and release with e-signature and an audit trail. Convert issues into CAPA or 8D linked to product and batch while tracking FPY, PPM and closures to cut defects, lower cost of quality, and boost customer satisfaction and compliance.
Benefits
- Automate 100% of Your Quality Frameworks
Compliance with leading quality standards like ISO 9001, ISO 13485, IATF 16949, AS9100 and more. - Centralize and Control Documentation
Store and manage all quality documents, including SOPs and work instructions.
- Faster review and release
Automate routing, approvals and notifications with e-signature and full traceability. - Lower cost of quality
Remove re-entry and duplicate tools, reduce scrap and rework, focus effort where it matters. - Advanced quality tools
Manage FMEA, APQP and PPAP to streamline risk, prove readiness and deliver right the first time.
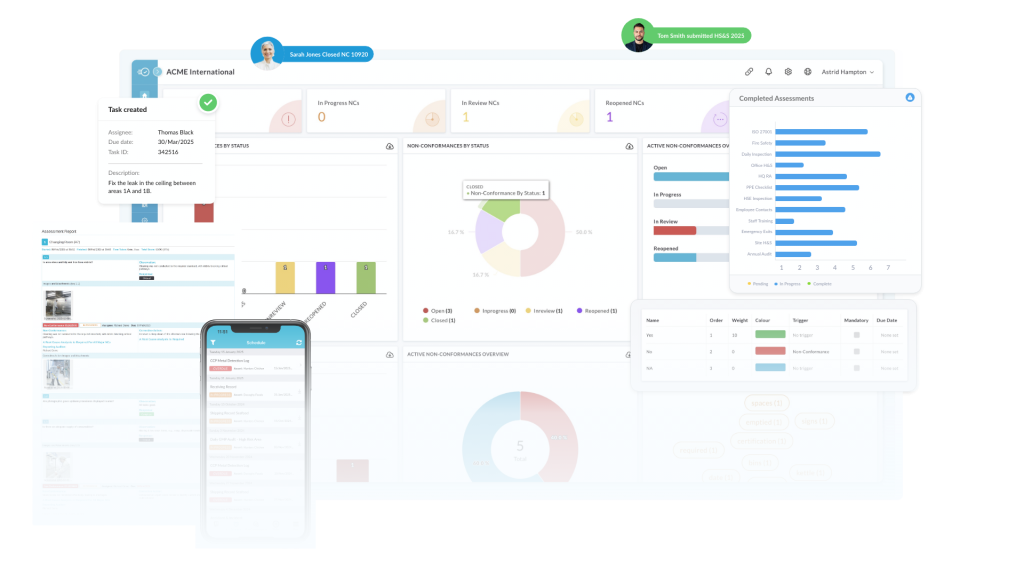
Quality responsibilities are scattered across teams, leading to missed inspections, repeat non-conformances, and a reactive approach to issues.
With Nulogy
Assign, track, and automate audits, inspections, and corrective actions to ensure accountability and proactive issue resolution.
Disconnected spreadsheets and manual reports make it impossible to get visibility into non-conformances and process inefficiencies.
With Nulogy
Centralize all quality data in one platform with AI-driven analytics for instant visibility and predictive insights.
Constantly changing regulations create compliance gaps, last-minute audit scrambles, and increased risk of fines and non-conformance.
With Nulogy
Digitize compliance tracking, document control, and audit management to stay continuously aligned with the latest regulations.
A lack of real-time supplier oversight increases the risk of defects, recalls, and non-compliance across the supply chain.
With Nulogy
Automate supplier audits, risk scoring, and compliance tracking to ensure consistent quality and reduce supply chain risks.
Industries We Serve
Understand and optimize your plant floor
We help manufacturers in the automotive industry solve their plant floor issues and deliver with excellence.
Manage quality control processes with ease
Nulogy’s software makes food & beverage QC processes a breeze. Sleep easy thanks to Nulogy’s digital traceability and recall capabilities.
Our purpose-built software helps metal fabrication manufacturers reduce downtime, increase availability, and ensure on-time delivery.
Nulogy’s software provides real-time visibility into contract packaging and manufacturing production processes and quality assurance.
Gain the real-time production visibility needed to monitor plastics manufacturing performance, machine health, and track key metrics.
See how Nulogy helps contract manufacturers, contract packagers, and raw and material suppliers work smarter and faster.
Proven Results
Nulogy QMS is trusted by leading manufacturers around the world. Our proven results:

Sysco Case Study: Transforming Food Safety & Quality with Nulogy QMS
Discover how Sysco Specialty Meat Group (SSMG) successfully implemented Nulogy QMS across 25 sites, increasing audit usage by 4x, improving yield, and streamlining SQF compliance. Learn how they recouped costs through raw material audits and ensured regulatory compliance with secure, read-only access for USDA, FDA, and CFIA.
SOME OF NULOGY’S CLIENTS
Trusted by Leading Manufacturers Worldwide – From Automotive to Heavy Machinery





Sysco Case Study: Transforming Food Safety & Quality with Nulogy QMS at SSMG
Discover how SSMG successfully implemented Nulogy QMS across 25 sites, increasing audit usage fourfold, improving yield, and streamlining SQF compliance. Learn how they recouped costs through Raw Material Audits and ensured regulatory compliance with secure, read-only access for USDA, FDA, and CFIA.

Learn more about our Quality Management Software
Discover how our QMS solution helps standardize processes, automate quality workflows, and shorten cycle times from nonconformance to release.
FAQ
Frequently Asked Questions
01 What is a Quality Management platform and how does Nulogy QMS support it?
Nulogy QMS is a single workspace to standardize checks, capture evidence, control documents, manage issues and actions, and report on performance. Nulogy QMS brings these functions together so teams run consistent processes and improve with data.
02 Can we standardize templates across sites while allowing local variations?
Yes. Govern master templates centrally, then allow site level variations under the same standard so every plant follows a common approach with local flexibility.
03 How does document control work?
Store SOPs, work instructions, and specifications in a controlled library with version history, read and understood tracking, and routed approvals so everyone uses the latest version.
04 How are non-conformances and CAPA managed?
Any finding can create an issue with linked actions, owners, due dates, reminders, escalations, and effectiveness checks. Everything remains traceable to the original record.
05 Does Nulogy QMS support review and release?
Yes. Findings, test results, and evidence flow through digital approvals with e-signatures and clear status by batch, line, and site to speed up release.
06 What analytics are available?
Live dashboards show leading and lagging indicators, trends, closure rates, and cost of quality. Data can be shared to enterprise BI tools such as PowerBI for board ready reporting.
07 Can we integrate with our existing systems?
Yes. Nulogy QMS can exchange key data with ERP, LIMS, MES, and data warehouses to reduce manual entry and keep records aligned across the organization.