Software
Quality Management System
Software
Quality Management System
quality & compliance, all in one app.
in real time.
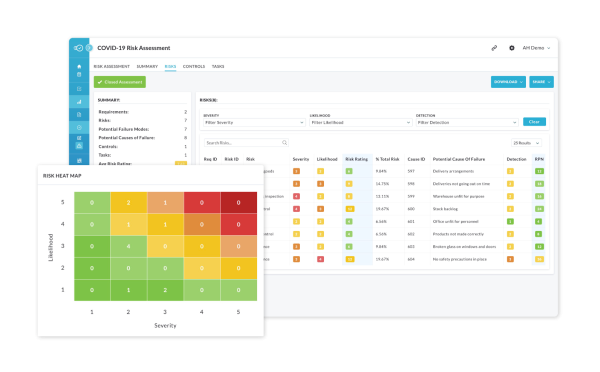
Smarter, easier risk, quality and compliance management
Turn Risk, Compliance, and Quality into a Competitive Edge.
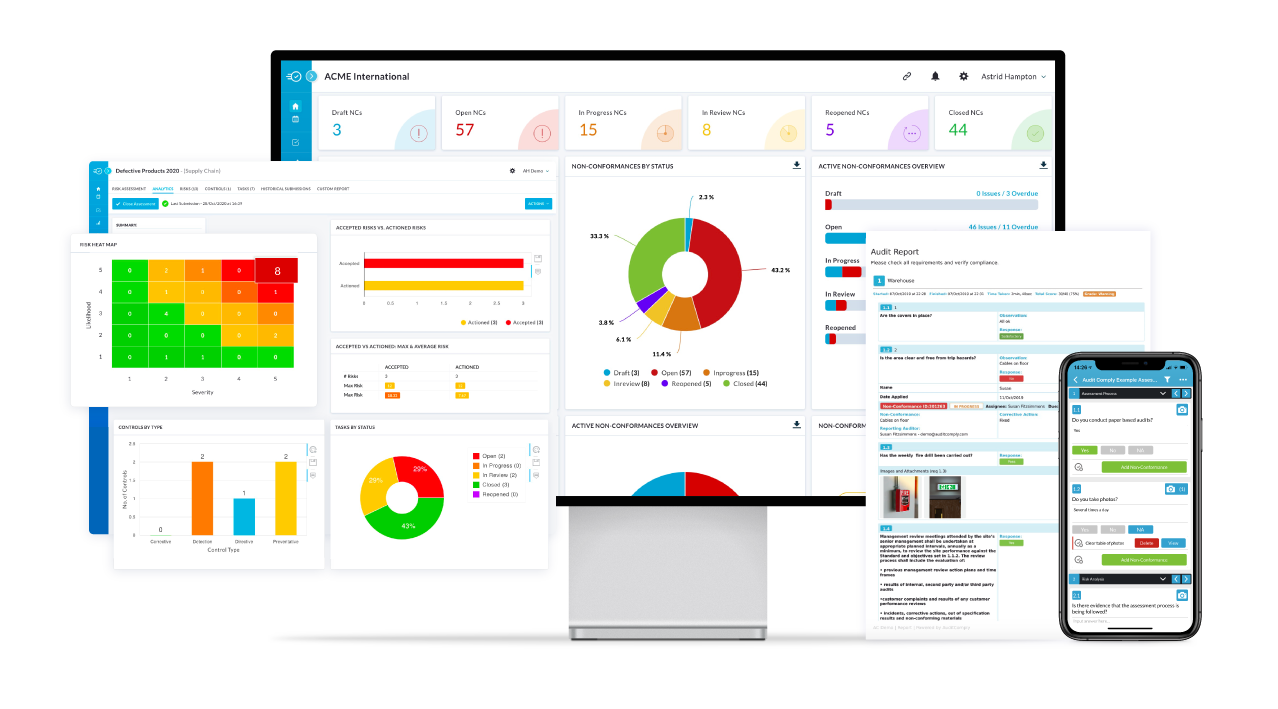
Nulogy’s Quality Management System is your one-stop shop for easily and quickly managing documents, workflows, and issues in risk, quality, and compliance.
Using our robust QMS and EHS modules, streamline your auditing and reporting processes, as well as compliance workflows, with support for leading quality standards such as ISO 9001, ISO 13485, IATC 16949, and AS9100.
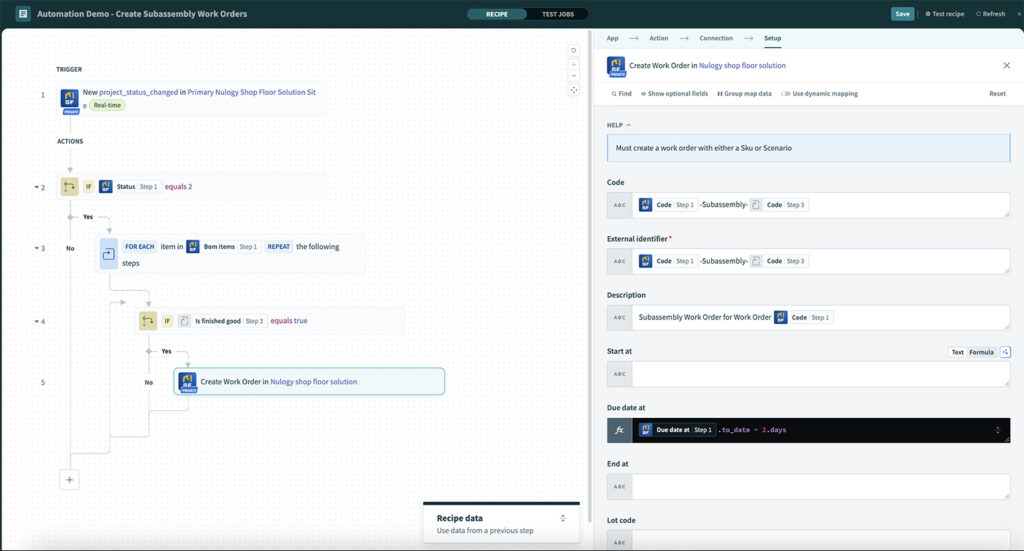
Build customizable frameworks, automate quality and compliance-related tasks, and capture evidence and documents in real time to lower costs, reduce errors and improve quality and safety.
Quality Management Benefits
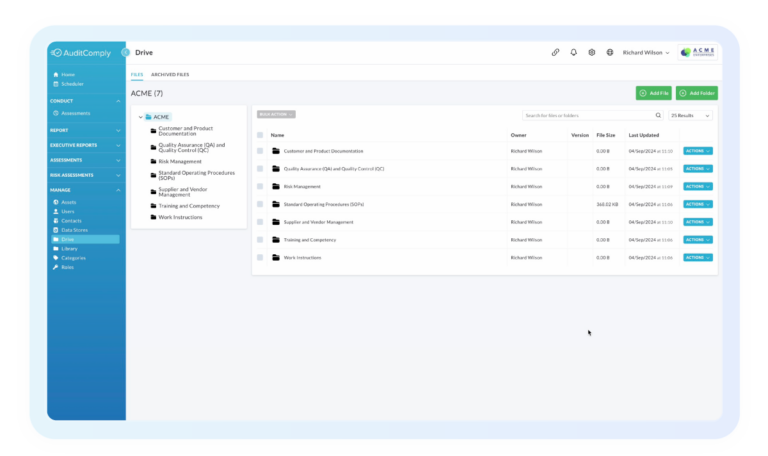
- Centralized quality data and document control:
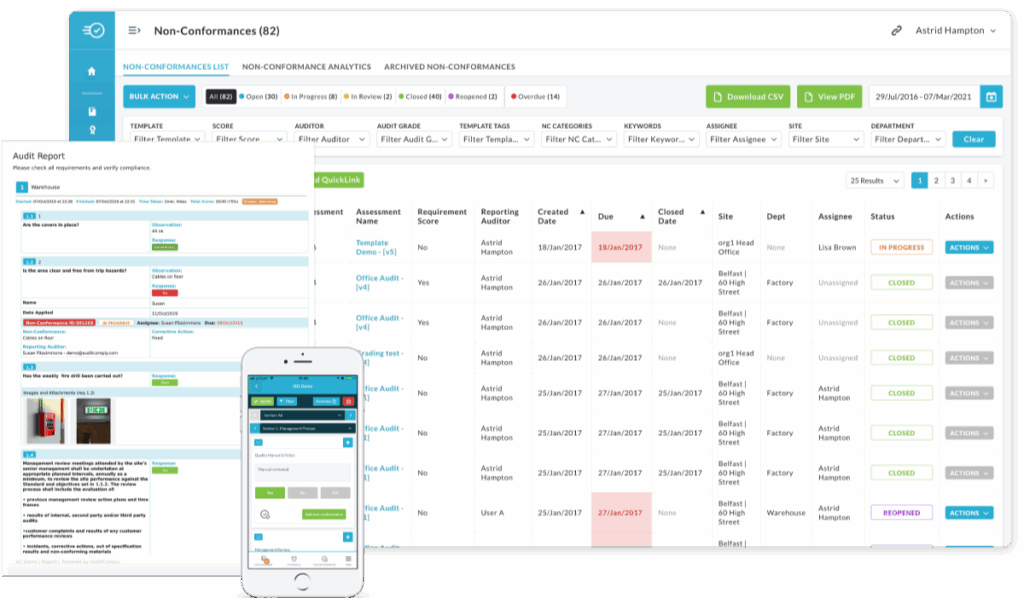
SOPs, specifications, batch records, test results and evidence in one secure library with access control, comments and version history, linked to audits. - Audits, gap analysis and CAPA to closure:
Plan audits, identify gaps, assign actions, track CAPA to completion and report trends for continuous improvement. - Faster review and release:
Automate routing, approvals, and notifications with e-signature and full traceability. - Advanced quality tools:
Manage FMEA, APQP, and PPAP to streamline risk, prove readiness and deliver right the first time. - Offline mobile capture:
iOS and Android with offline support to audit, inspect, and attach photos and evidence on the line.
Compliance Management Benefits
- Near Miss, Incident and Hazard Reporting: Empower every worker to report hazards, near misses, injuries and environmental events from any device, in real time. Capture photos, time and location, trigger alerts, automate triage and corrective actions, and trend data to prevent recurrence.
- EHS Audits and Inspections: Standardize safety walks, equipment checks and environmental inspections with smart checklists linked to risks, controls and frameworks such as ISO 45001, OSHA and HSE guidance. Drive consistent scoring, evidence capture and overdue action tracking.
- Documents, Training, Permits and Contractors: Control policies, SDS and procedures with versioning. Schedule toolbox talks and refreshers, verify competencies, manage permits to work such as confined space and hot work, and monitor contractor pre-qualification, insurance and performance with real time dashboards.
Popular Compliance & Quality Frameworks
Effortlessly manage compliance frameworks, streamline evidence collection, and ensure continuous compliance with Nulogy.
ISO 9001 Quality Management
ISO 9001 is the world’s most widely recognized standard for establishing and maintaining a Quality Management System (QMS). It provides a structured framework for organizations to deliver consistent quality, meet regulatory requirements, and enhance customer satisfaction. Adopted across industries such as aviation, construction, automotive, and health sciences, ISO 9001 is often a prerequisite for doing business.
IATF 16949 Automotive Quality
IATF 16949 is the global standard for Quality Management Systems in the automotive industry. Streamline your automotive quality processes with a solution designed to enhance compliance, mitigate risks, and drive improvement. Focus on defect prevention, supply chain optimization, and continuous improvement to reduce risks, boost efficiency, and ensure high-quality products.
BRCGS Food Safety
Manage critical control points, monitor hazards in real time, and ensure compliance with BRCGS. Automate data collection, streamline corrective actions, and eliminate paperwork with a centralized platform. With real-time alerts and digital record keeping, Nulogy enhances food safety, reduces risk, and keeps you audit-ready.
HACCP Food Safety
Streamline the HACCP process, helping organizations implement, monitor, and maintain food safety protocols efficiently. Create templates and workflows that support a continuous cycle of proactive practices and safety checks, ensuring risks are identified and mitigated at every stage of food production.
Quality responsibilities are scattered across teams, leading to missed inspections, repeat non-conformances, and a reactive approach to issues.
With Nulogy
Assign, track, and automate audits, inspections, and corrective actions to ensure accountability and proactive issue resolution.
Disconnected spreadsheets and manual reports make it impossible to get real-time visibility into non-conformances, trends, and process inefficiencies.
With Nulogy
Centralize all quality data in one platform with AI-driven analytics for instant visibility and predictive insights.
Constantly changing regulations create compliance gaps, last-minute audit scrambles, and increased risk of fines and non-conformance.
With Nulogy
Digitize compliance tracking, document control, and audit management to stay continuously aligned with the latest regulations.
You struggle to manage EHS workflows with a jumble of paper audits, Word checklists, and Excel trackers.
With Nulogy
Standardize audits and inspections with a drag-and-drop Template Builder, ready-to-use EHS framework library, and recurring schedules.
Your near miss reports get lost in notebooks and inboxes, leading to slow responses.
With Nulogy
Mobile near miss reporting, instant capture with photos and notes, offline on site, and automatic routing to the right people.
Evidence is scattered across paper permits and static asset lists.
With Nulogy
Centralize evidence, permits, and assets with a site to line to equipment hierarchy, scheduled checks, approvals, and full history.
Proven Results
Nulogy Quality Management System is trusted by leading manufacturers worldwide. Our proven results:

Sysco Case Study: Transforming Food Safety & Quality with Nulogy
Discover how Sysco Specialty Meat Group (SSMG) successfully implemented Nulogy QMS across 25 sites, increasing audit usage by 4x, improving yield, and streamlining SQF compliance. Learn how they recouped costs through raw material audits and ensured regulatory compliance with secure, read-only access for USDA, FDA, and CFIA.
SOME OF NULOGY’S CLIENTS
Trusted by Leading Manufacturers Worldwide – From Automotive to Heavy Machinery





Sysco Case Study: Transforming Food Safety & Quality with Nulogy QMS at SSMG
Discover how SSMG successfully implemented Nulogy QMS across 25 sites, increasing audit usage fourfold, improving yield, and streamlining SQF compliance. Learn how they recouped costs through Raw Material Audits and ensured regulatory compliance with secure, read-only access for USDA, FDA, and CFIA.

Learn more about our Quality Management Software
Discover how our QMS module helps standardize processes, automate quality workflows, and shorten cycle times from nonconformance to release.