Nulogy’s Quality Management System platform helps manufacturers and distributors control risk, strengthen compliance, and elevate quality across every site, line, and supplier in one connected real-time system.
Quality managers have one workspace to control specs, documents, issues and manage supplier performance. Enforce line checks, capture evidence on any device, and route review and release with e-signature and an audit trail. Convert issues into CAPA or 8D linked to product and batch while tracking FPY, PPM and closures to cut defects, lower cost of quality, and boost customer satisfaction and compliance.
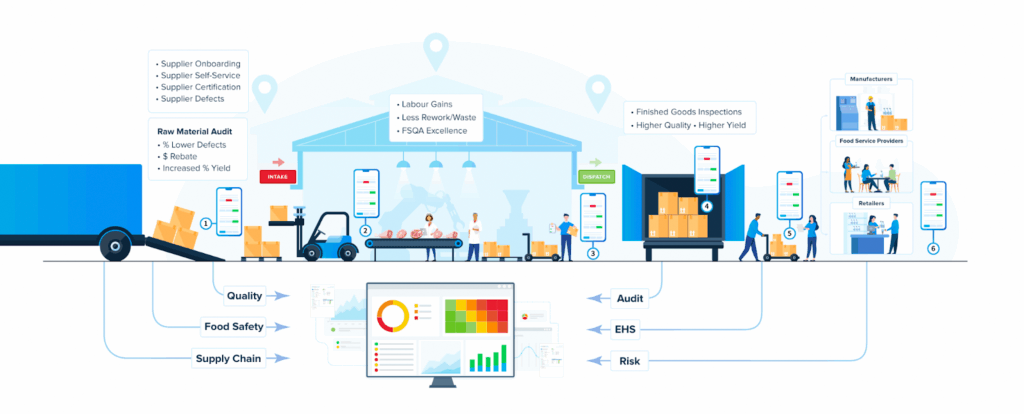
Industry-leading brand manufacturers, contract packagers and contract manufacturers leverage Nulogy’s software to optimize order planning and fulfillment, and work together to execute perfectly.
Centralize and govern all specs, SOPs, and quality records with full version control
Protect people and operations with proactive, data driven safety management.
Automate standards, audits and controls to stay audit-ready, with full traceability.
Improve relationships & collaboration with dedicated supplier portals.
Drive consistent, real-time quality across every product, line & site
Nulogy QMS is a single workspace to standardize checks, capture evidence, control documents, manage issues and actions, and report on performance. Nulogy QMS brings these functions together so teams run consistent processes and improve with data.
Yes. Govern master templates centrally, then allow site level variations under the same standard so every plant follows a common approach with local flexibility.
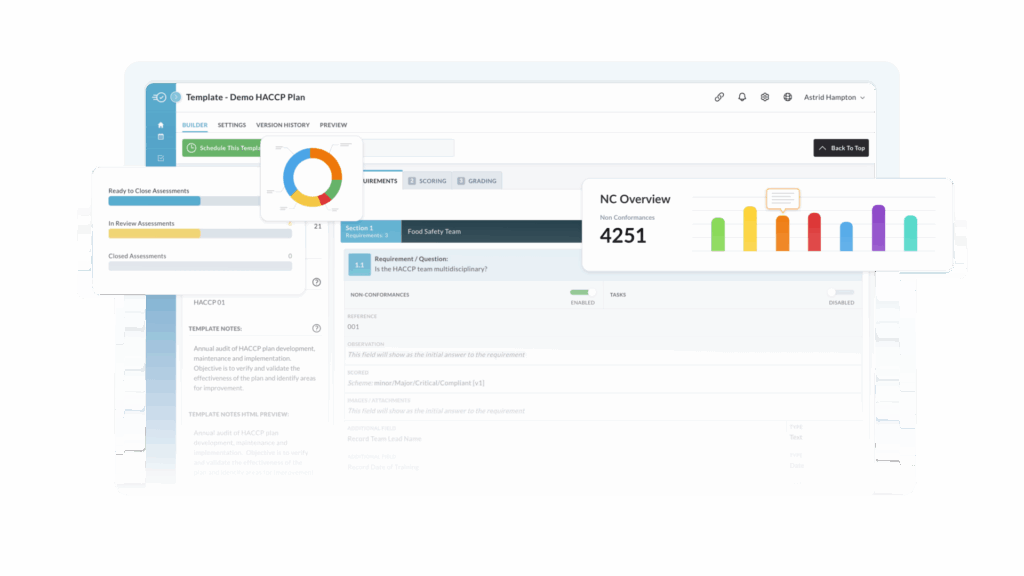
Store SOPs, work instructions, and specifications in a controlled library with version history, read and understood tracking, and routed approvals so everyone uses the latest version.
Any finding can create an issue with linked actions, owners, due dates, reminders, escalations, and effectiveness checks. Everything remains traceable to the original record.
Yes. Findings, test results, and evidence flow through digital approvals with e-signatures and clear status by batch, line, and site to speed up release.
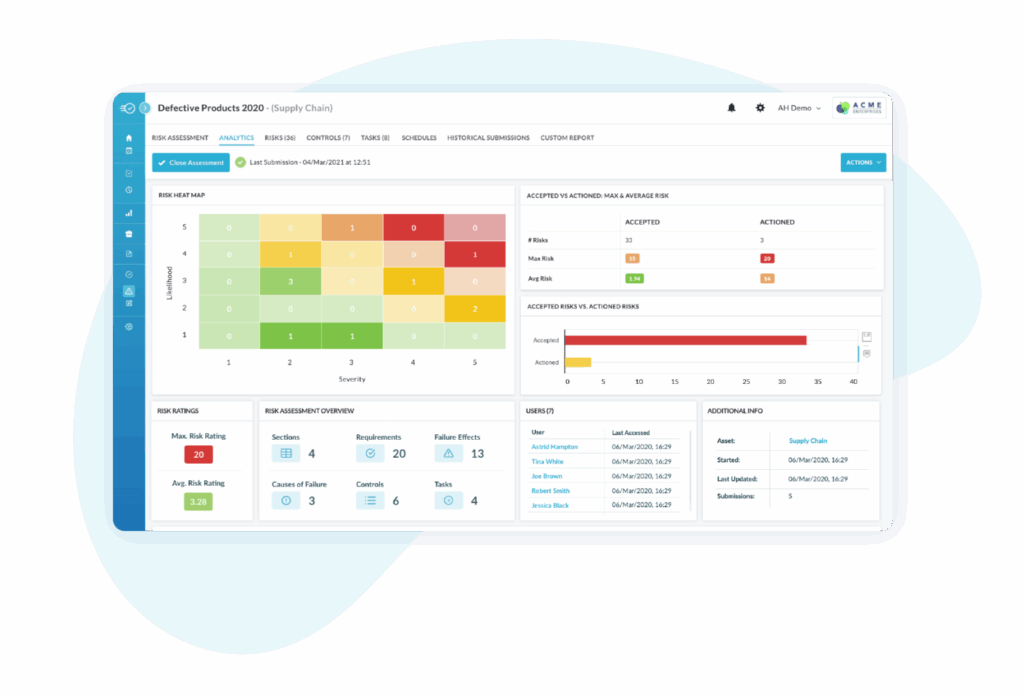
Live dashboards show leading and lagging indicators, trends, closure rates, and cost of quality. Data can be shared to enterprise BI tools such as PowerBI for board ready reporting.
Yes. Nulogy QMS can exchange key data with ERP, LIMS, MES, and data warehouses to reduce manual entry and keep records aligned across the organization.